Equipment Validation
Everything you ever needed to know about equipment validation
What is equipment validation?
Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety requirements. The equipment validation process is usually done to meet quality management standards, such as ISO 13485 and ISO 9001.
Businesses typically employ third-party experts to develop and implement a validation plan that helps reduce time and expenses for personnel and other overhead costs. Moreover, validation experts can quickly perform validation activities, and interpret potentially vague regulations, reducing misinterpretation risks.
Equipment validation is a fundamental component of good practices (GxP) — including Current Good Manufacturing Practice (CGMP) Regulations — and the ideal way for high-risk industries to ensure dependable product quality.
Validation is mandatory for certain quality standard certifications (like ISO 13485) that regulators meticulously investigate during asset audits.
The Essential Guide to CMMS
Download this helpful guide to everything a CMMS has to offer.

Which industries implement equipment validation?
Many industries use equipment validating processes, including — but not limited to:
- Biotechnology manufacturing and distribution
- Research facilities and laboratories
- Automotive manufacturing
- Computer software development
- Environmental monitoring.
Due to consumer safety concerns, any industries regulated by GxP standards must undergo formal validation. Such industries include:
- Medical devices and pharmaceuticals (development, testing, and distribution)
- Food storage and distribution
- Aerospace manufacturing and maintenance.
In addition, the biotechnology industry has specific guidelines which are noted on the FDA website
Why is validation important?
The equipment validation process requires lots of money, time, planning, and knowledge. Nonetheless, it is essential as it guarantees reliable product quality, resulting in fewer recalls and higher customer satisfaction.
Violations can significantly affect companies and consumers, resulting in:
- Revenue loss
- Large product recalls
- Costly lawsuits
- Government fines.
Noncompliance with FDA regulations can hinder the authorization process and delay release of certain products such as medicines, new supplements, and the like.
Companies often fail to implement good practices — created for assuring consumer safety and lowering risk — with violations becoming increasingly common over the past decade.
For instance, in 2018 the pharmaceutical industry received five times the number of warning letters from the FDA for violations, compared to the number of warnings received in 2015.
Guide to Moving from Reactive to Preventive Maintenance
Want to transition away from costly reactive maintenance but don't know where to start? This guide has everything you need to know.

Phases of the equipment validation process
The validation process includes three main phases:
- Pre-validation
- Process validation
- Validation maintenance.
Each of these phases has sub-stages or types of equipment validation which we’ll discuss later in the article.
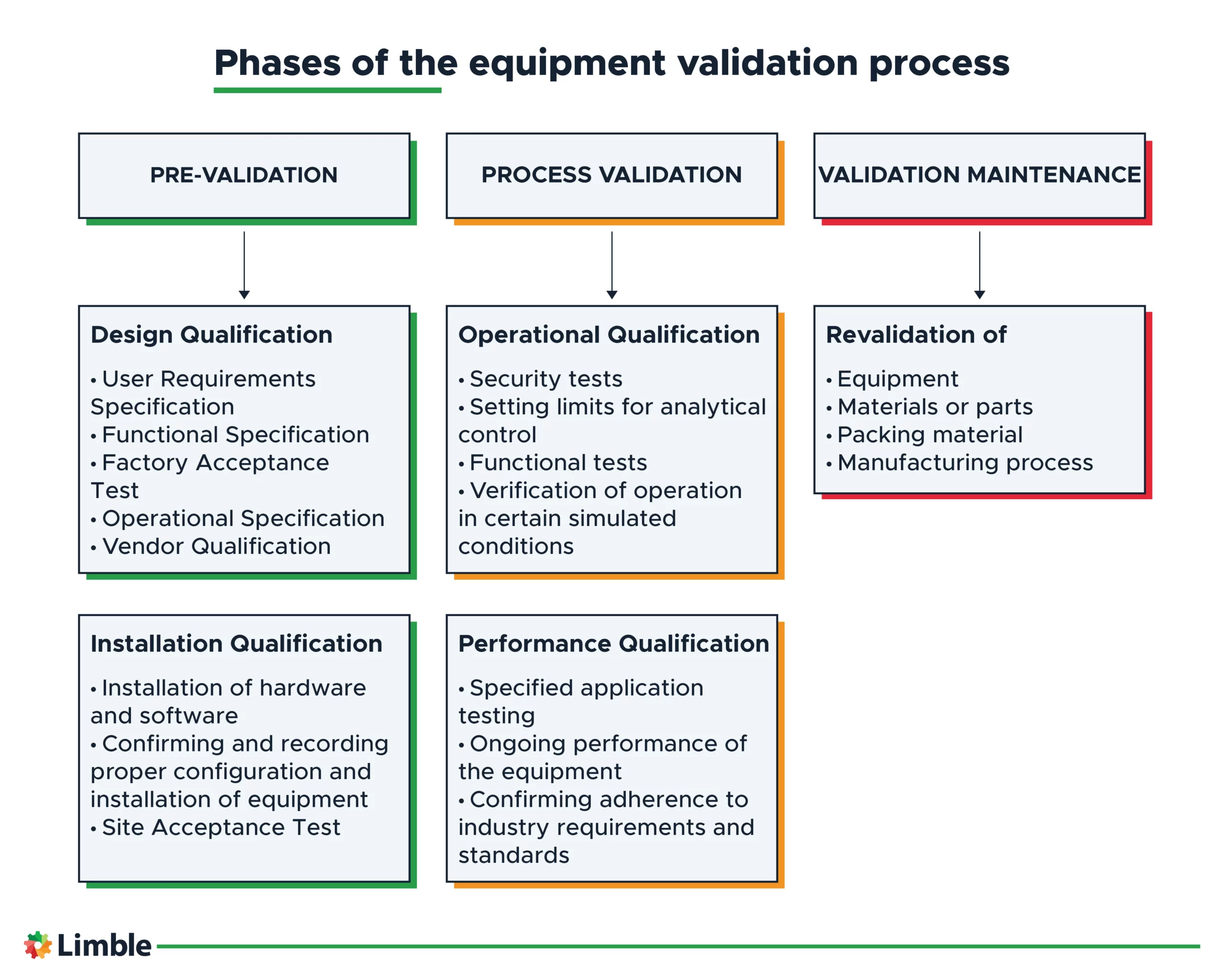
1. Pre-validation of equipment
The first phase of the process is pre-validation, which defines whether the equipment can operate within the desired environment as intended per the user’s requirements. You’ll have to consider the needs of the overall process to determine the equipment specifications.
The pre-validation phase has two validation stages:
- Design Qualification (DQ)
- Installation Qualification (IQ).
Design Qualification
Design qualification is the process of demonstrating that the equipment functions appropriately and within prescribed parameters. The DQ stage may include several validations, such as:
- User Requirements Specification (URS)
- Functional Specification
- Factory Acceptance Test (FAT)
- Operational Specification
- Vendor Qualification
Installation Qualification
Installation qualification ensures equipment is installed properly and the environment is appropriate for equipment use.
Manufacturer specifications, such as environmental requirements (humidity and temperature), or electrical requirements (frequency and voltage ratings), are referenced to execute this stage.
The IQ stage may include the following:
- Installation of hardware and software
- Confirming and recording proper equipment configuration and installation
- Site Acceptance Test (SAT).
2. Process validation of equipment
This phase involves running repeated trials, mimicking the required performance of the equipment to confirm that it consistently complies with the predetermined conditions for operation and quality.
The process validation phase is executed in two stages:
- Operational Qualification (OQ)
- Performance Qualification (PQ).
Operational Qualification
This step is an inspection of the overall equipment functionality, which includes testing general operations and verifying proper function within the specified parameters.
The goal is to document that the equipment indeed performs according to the required specifications. The OQ stage typically includes:
- Security tests
- Setting limits for analytical control
- Functional tests
- Verification of operation in certain simulated conditions.
Performance Qualification
This stage demonstrates that the equipment’s functions, per requirements, are relevant to routine use. In this stage, equipment must pass distinct tests, proving it will dependably perform its daily tasks on an ongoing basis.
The PQ stage normally includes the following:
- Specified application testing
- Ongoing performance of the equipment
- Confirming adherence to industry requirements and standards.
3. Validation maintenance
The third phase of the process is known as post-validation maintenance or revalidation. If the earlier steps don’t lead to the desired outcome, you need to make the necessary changes to address why.
The validation maintenance phase implements constant equipment monitoring, running performance tests, and verifying that the overall function produces the intended result.
Parameter windows are refined, and equipment is re-qualified and recertified to maintain validation.
In this case, a range of alterations may be required depending on the specific performance issue, and may involve making changes to the following before re-qualification:
- Equipment
- Materials or parts
- Packing material
- Manufacturing process.
Equipment validation best practices
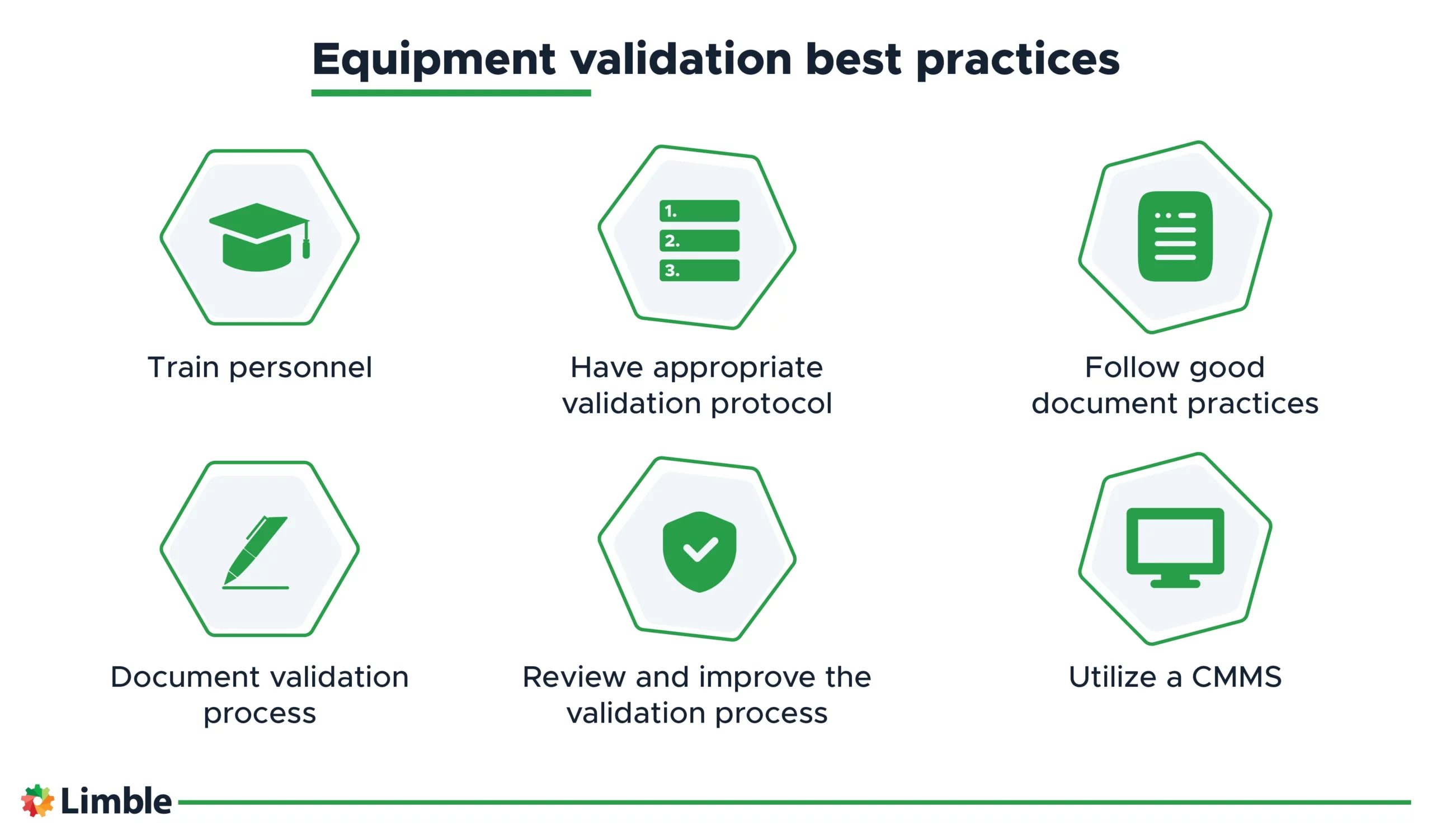
To ensure an effective equipment qualification process that meets the required quality standards, follow the best practices below. This will help reduce equipment downtime, prevent product recalls, and avoid costly regulatory fines.
- Train personnel: Ensure personnel involved in equipment validation are competent for the job. This includes training related to validation plans, protocols, and procedures.
- Have an appropriate validation protocol in place: A document that outlines the process that needs to be validated, including the production equipment and the methodology for conducting the validation.
- Follow good documentation practices: Adhering to good documentation practices ensures that your documentation — and your products — meets industry standards and fulfills quality assurance, regulatory compliance, and other legal responsibilities.
- Record the whole validation process: Documentation provides a complete record of the equipment validation process and evidence that the process was conducted according to the defined protocols and procedures. It includes performed tests, deviations, changes, and corrective actions taken.
- Review and improve the validation process: With continuous improvement, you’ll ensure that the equipment remains in compliance, produces high-quality products, and remains competitive in the market.
- Use a CMMS: A CMMS system will provide real-time data on equipment performance and keep a record of all maintenance activities and related documents (protocols, standard operating procedures (SOPs), work orders related to equipment validation, etc.). It will ensure the equipment is maintained according to the manufacturer’s recommendations and demonstrate compliance with regulatory requirements.
Want to see Limble in action? Get started for free today!
Validate your equipment
Equipment validation is an important process that ensures the equipment used in highly regulated industries:
- Operates effectively
- Meets required standards
- Produces high-quality products.
If you are under the scrutiny of external auditors, it pays to follow equipment validation best practices.
To find out more ways to improve equipment performance and equipment reliability, keep browsing the Limble blog.