Streamline Compliance
No More Painful Audits.
Stay Compliant with Ease
Pass ISO audits, streamline OSHA inspections, remain 21 CFR compliant, and more.

41%+
Increase in productivity
37%+
Drop in
downtime
$145M
Saved in annual parts spend






Learn How Limble Makes Audits Easy
Limble is trusted by 50,000+ maintenance professionals
Audits
Reduce the time you spend on audit prep and pass inspections with ease by keeping performance and maintenance history accessible to pull reports in seconds
- Create and track work orders with all the relevant information on your assets
- Track maintenance tasks and achieve higher safety and compliance rates
- Checklists to ensure all steps in a task are complete and documented
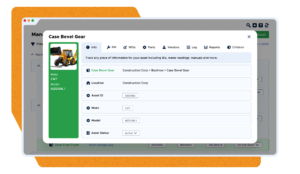

“Auditors were satisfied very quickly” – Corey Mince, Process Engineer, Spectrum Solutions
Compliance
Track your team, schedule recurring inspections, and maintain an auditable record to stay compliant with regulations like Title 21 of CFR
- User-friendly preventive maintenance templates with SOPs, checklists, photos and more
- Easily set up calibration schedules for all of your calibrated equipment. Retain a complete log of all calibration certificates and past results
- Track completion rates on PMs and work orders in one place, in real-time
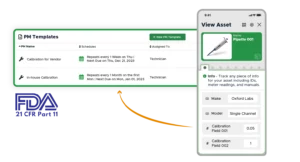

“Limble simplifies life as a maintenance supervisor!” – Robert Stricklin, Maintenance Supervisor, City of Lompoc Water Treatment Plant
Documentation
Consolidate all of your essential documentation to keep your team informed, productive, compliant, and safe
- Build work orders with complete asset information, due date, priority, and more
- Checklists to ensure all steps in a task are complete and documented
- Access important documents like repair manuals or warranty information
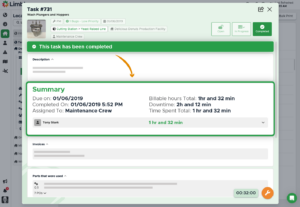

“Being able to put everything in Limble and have that accessible out on the floor has been huge.” – Matt Burtz, Maintenance Director, Preferred Popcorns
Streamline Communications
Keep your full maintenance team connected, productive, and secure with a centralized collaboration platform
- Real-time organization, communication, and access to information
- Anyone can scan a QR, scan a barcode, or visit a URL to submit an issue
- Automatically update work requesters on the status of their tickets via simple, customizable workflows
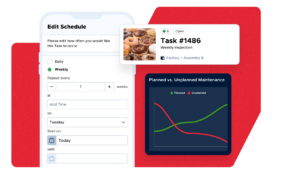

“From the stone age to the modern age with our work order systems.” – Todd Rainwater, Director of Facilities and Operations, Hood River County School District
Compare Pricing
Basic
Standard
Premium+
Enterprise

Spectrum Solutions
Medical device manufacturer, Spectrum Solutions, uses Limble to meet strict FDA guidelines, making audit stress a thing of the past.
FAQ
What is CMMS software?
CMMS (Computerized Maintenance Management System) software helps businesses manage, automate, and streamline all of their maintenance operations. Learn more about CMMS.
Who uses CMMS software?
CMMS software is used by anyone who manages maintenance — facility managers, operations managers, and asset managers, and more — to manage assets, schedule maintenance, and ensure safety. It is widely employed in industries including manufacturing, education, energy & utilities, food & beverage, and many more to maintain infrastructure and manage resources effectively.
Is Limble Mobile CMMS app user friendly?
Limble is consistently rated Easiest-to-Use CMMS on review sites like G2, Capterra, and Software Advice. And our customers agree. With our mobile CMMS app, teams experience 30%+ better productivity, on average, requiring little to no training or ramp-up time. Our CMMS app can travel with your team, no matter where they go! Visit our App Store or Google Play for more information.
Can I connect to other systems?
Limble provides seamless, pre-built CMMS Integrations with the most widely used software systems. That means you won’t need help from a developer or your IT team to get started. Learn more about our integrations.
How secure is the Limble CMMS platform?
At Limble, our world-class data security practices ensure your account information is safe. We use state-of-the-art technologies and industry best practices to maintain a secure infrastructure, including SOC-II Type II certification, regular penetration testing, and continuous security training for our staff.
TRUSTED BY 50,000+ PROFESSIONALS AROUND THE WORLD
All-in-one CMMS for all your Maintenance Needs
Work Orders & Requests
Organize work orders, PMs and other tasks for your entire team. Easy-to-use mobile apps your technicians will actually use.
See Work Order SoftwarePreventive Maintenance Programs
Automate preventive maintenance scheduling, build simple checklists, and analyze team performance and efficiency.
See Preventive Maintenance SoftwareAsset Management
Intuitive and flexible asset management features make it easy to manage your assets, whether you have 10 or 10 million.
See Asset Maintenance Management SoftwareSpare Parts Inventory
Eliminate the guesswork and reduce the amount of time and money spent searching and managing your parts inventory.
See Parts Inventory Software

